Dalian Xueao plans to build Asia's largest industrialized Cordyceps sinensis base
For centuries, Cordyceps sinensis has enjoyed a high reputation at home and abroad as a valuable nourishing medicine. However, because it only grows in the special climate and eco-geographical environment of the snow-covered plateau in China, the natural resources are scarce. As a result, research and development of Cordyceps mycelia have been carried out in various regions throughout China. According to Mr. Lei Ming, Chairman of Dalian Xueao Company, the company joined the research and development of artificial cultivation of Cordyceps militaris from 1996. It not only successfully isolated high-quality Cordyceps sinensis fungal strains from fresh wild Cordyceps sinensis collected from the Sichuan Plateau. In addition, a complete set of technological processes such as separation, purification, rejuvenation, preservation and cultivation of strains and deep fermentation was solved to simulate the special growth environment of wild Cordyceps sinensis and successfully cultivated the drug intermediates of Cordyceps sinensis mycelium. Recognized by an authoritative department, the technology used in this project is the first in the country, and it is at the leading level in the world. Its product quality and yield rank the leading position in the world. Its effective composition has reached the national drug standards promulgated by the State Food and Drug Administration. It is understood that the project's total investment will reach 210 million yuan, according to GMP standards to build first-class, large-scale modernization of Cordyceps sinensis fermentation process production lines, drug intermediates production lines, new drugs and health food series production lines and high-tech research and development centers and supporting facilities. At present, the first phase of the project will be completed and put into operation in August, and the second phase will be completed by August next year. By then, the annual production of Cordyceps sinensis mycelia will reach 345.6 tons, and it is expected that all exports will earn 55.3 million US dollars for the country each year. The project has applied for international patents.
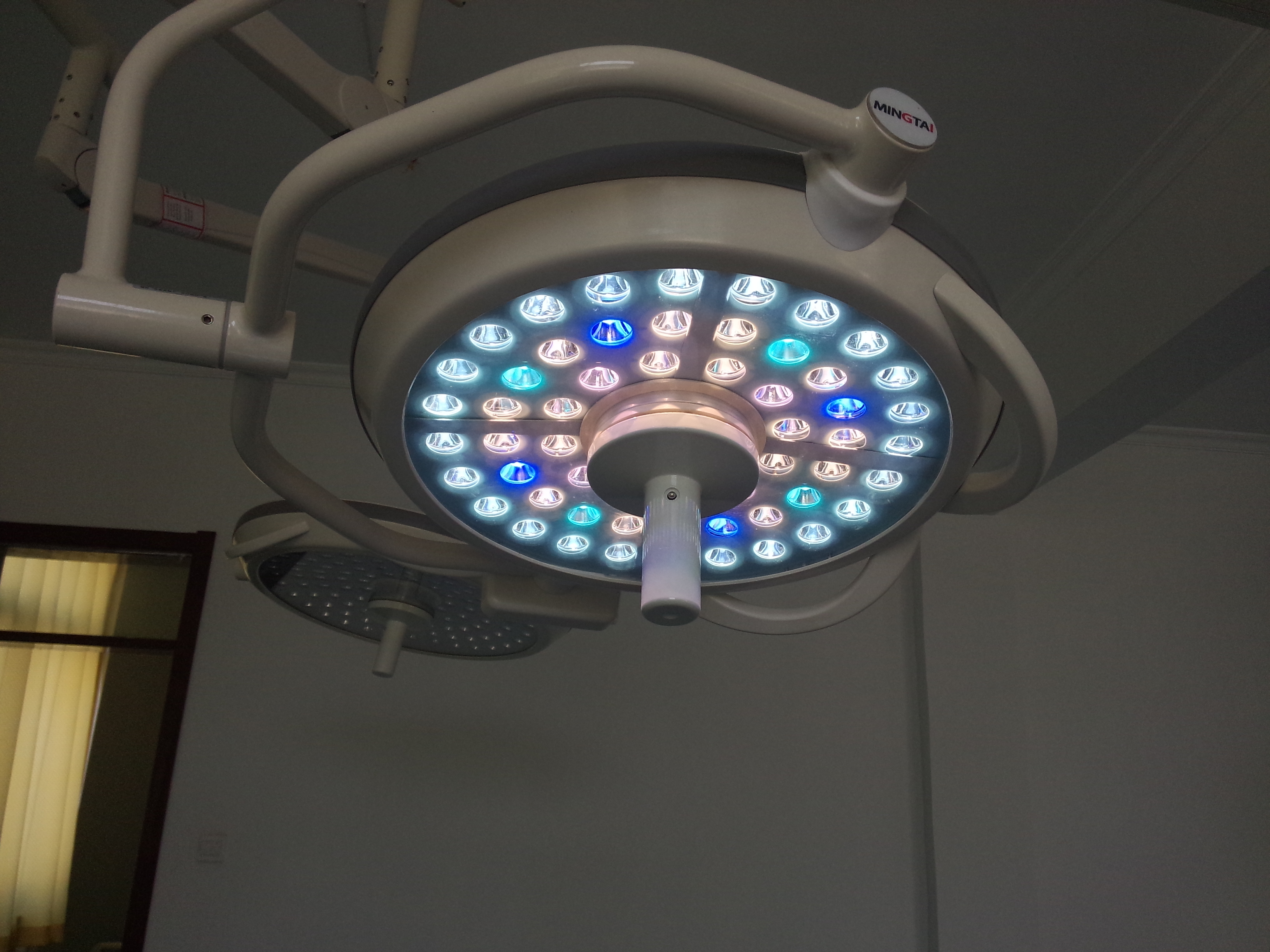
Mingtai Operating Lamp adopts international advanced technology, it can support the best shadowless condition for surgery. With more than 10 years experience, mingtai shadowless lights have different types to meet different requirements. Mingtai surgery lamp equips with world famous LED bulb, it lifetime can reach 60,000 hours, and it temperature rise is very low. Each LED bulb is controlled by special circuit chip, any
group failure will not affect the normal use of shadowless light. Mingtai operation light equips with petented disc spring brake syste, preventing drifting.
For more information about Operating Light, Operating Table, Hospital Bed and Medical Pendant please visit website.
Operating Lamp Operating Lamp,Veterinary Surgical Light,Medical Led Light,Shadowless Lamp Shandong Mingtai Medical Equipment Group Co., Ltd , http://www.mingtaic.com