Domestic and foreign cancer big data company inventory: Who can solve the pain point of pharmaceutical companies?
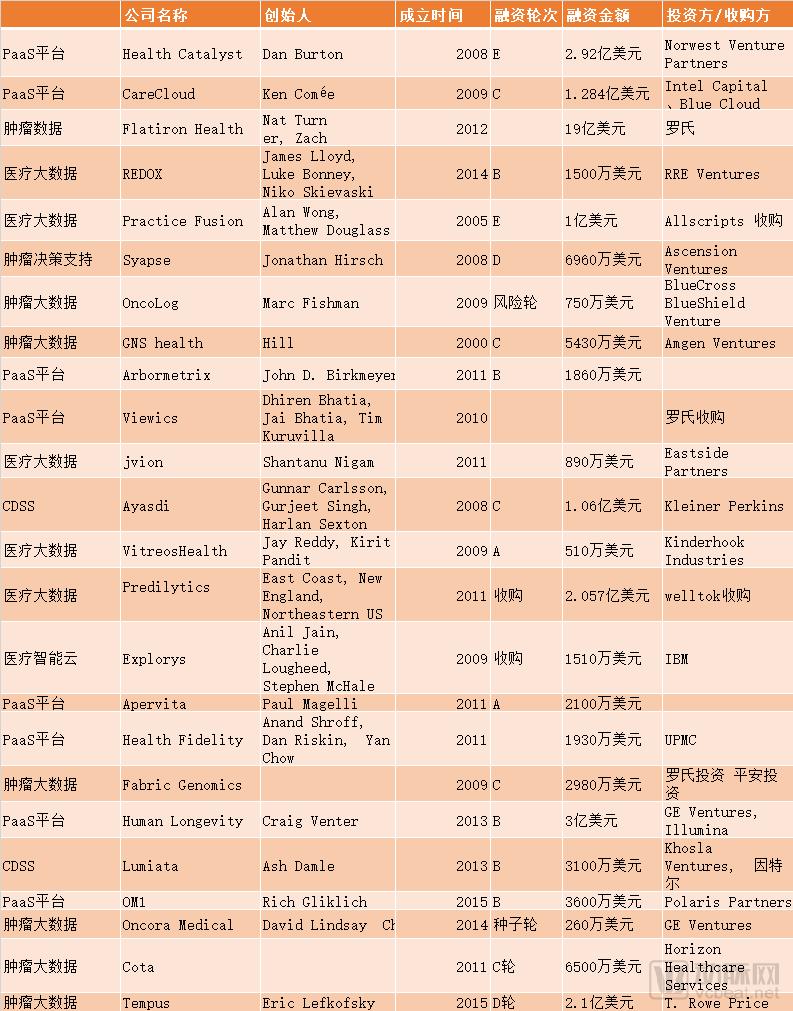
Medical clinical data is like a treasure buried in the mountains. Although there are gold mines in the mountains, if there are no good mining equipment, large gold mines can only be the Gobi that the grass mustard. Clinical big data has a huge impact on pharmaceutical companies, health care providers, medical payers and patients. But now because of the low data density, the data is in an island state, and a large amount of data is not connected to the patient's long-term follow-up, it has not been used. With the unprecedented growth of medical data, many companies are using analytical tools, artificial intelligence and machine learning technologies to obtain data-driven decision support to reduce healthcare costs, increase hospital revenue streams, develop personalized medicines, and patient care. management. Now, with the entry of more paying parties, and the role of big data applications at the medical level and promoting better medical outcomes, it is becoming more and more obvious. We can see that the new generation of gold miners are already at their peak. The data also supports this point. According to a report by BIS Research entitled "Global Medical Market Big Data Analysis and Forecast, 2017-2025", the size of the big data market in the medical field is estimated to be $14.25 billion in 2017. It is expected to grow by more than $68.75 billion by the end of 2025. What the company has made any breakthroughs in the field of healthcare big data, enterprise and innovation is a breakthrough from which direction to resolve medical problems in large data applications on large medical data this gold mine. The arterial network was counted. Medical big data solution The reason why the medical big data market is so welcoming is that it can meet many requirements. For pharmaceutical companies, a large amount of data from patients can promote real-world research and solve the needs of pharmaceutical companies. Pharmaceutical companies must submit drug safety testing data after the drug is marketed, otherwise they will face the risk of delisting. Real-world research can meet the compliance requirements of pharmaceutical companies. Expand drug accessibility and market capacity. For example, a drug suitable for patients with terminal cancer. Through real-world data research, it is proved that the effectiveness of drugs can turn second-line drugs into first-line drugs, and first-line drugs are pushed to earlier and broader markets to expand the market capacity of drugs. In the development of new drugs, due to the development of precision medicine and personalized medicine. Medical big data can provide direction for new drug development. Through observational research on real-world data, we can understand the incidence, prevalence, disease burden, complications, diagnosis and treatment of diseases, etc., so as to know the important clinical problems that need to be solved. In addition, RWE may also provide clues about pathogenesis to identify potential therapeutic targets. Big data can also solve patient recruitment problems. The clinical trial probability passed by the FDA is approximately 7%. Approximately one-third of Phase III clinical studies were terminated due to patient recruitment difficulties. Data from IQVIA showed that 37% of clinical trial sites were under-recruited. Pharmaceutical companies or CROs can't match the right patients. With the advent of precision medicine and personalized medicine, there should be fewer and fewer people. Take the tumors that all major pharmaceutical companies must strive for. As more and more molecular subtypes are discovered, the number of patients in each group is decreasing. It is becoming more and more difficult for traditional RCTs to find suitable subjects. With the investment of a large number of new anti-tumor drugs, the demand for evaluating the effects of new drugs is increasing. It is only in the Roche family that 488 tumor trials are underway in 2017. Second, the FDA is also demanding increased patient diversity, both in clinical trials and after marketing. Experience has shown that the more diverse the patients pooled in clinical trials, the more often the products are safer and more efficient. Finding enough clinical trial patients within the right range is not easy without big data. Previous RCT control trials have strict exclusion and inclusion criteria for patients, and patient homogeneity is severe. It also prevents many patients from accessing clinical trials. After the drug is marketed, a large amount of medical big data can also help expand the use of drugs. In the development of new drugs, less than one-thousandth of the active compounds can enter the clinical phase I trial, and it is found that the new role of existing drugs can be said to be a profit. However, in the past, pharmaceutical companies could only find new indications after a long period of time through expensive RCT (Clinical Randomized Controlled Trials) trials. The registration fee is very high and the risk is high. In effect, RCT is a highly specialized scenario, and real-world research is subject to long-term tracking of a large number of subjects and fairly large patient samples, with a focus on clinically meaningful outcome measures. It is worth mentioning that the application of real world research in the clinical research of traditional Chinese medicine has also attracted people's attention in recent years. Real-world research has broken through previous randomized control trials requiring simple and clear interventions, successful control measures, and highly homogeneous research populations. Real-world research is patient-centered and focuses on overall efficacy in evaluation indicators. More suitable for the characteristics of Chinese medicine. A large amount of real-world data can not only solve the urgent needs of pharmaceutical companies, but also solve the doctor's research needs after real-world data reaches the level of evidence, help doctors save the time cost of the papers, and get better research results. For the hospital, the application scenario is more abundant, making hospital management more efficient and service more human. Use big data to evaluate the medical practice process. For example, vinorelbine tartrate is a chemotherapy drug for the treatment of lung cancer and breast cancer. It has two routes of administration, oral and intravenous. By comparing and analyzing patients who received different routes of administration, it was found that oral administration can greatly shorten patient waiting time and improve the efficiency of receiving the chemotherapy center. Although medical big data is a gold mine, it is not so easy to develop it. In terms of policy, the introduction of policies is often accompanied by supervision and promotion. In 2016, the United States promulgated the "21st Century Treatment Act" proposed two concepts: RWD (real world data) and RWE (real world evidence). There is a requirement for health big data to reach the level of medical evidence. Health Big Data wants to reach the level of medical evidence, with a certain degree of data relevance and reliability. Data traceability also needs to be guaranteed, and it must also be guaranteed that there is no privacy violation. In 2016, the EU introduced the most stringent data protection regulations, the General Data Protection Regulation. It stipulates the transparency of personal data processing, the principle of minimum data collection, and gives the data subject the right to withdraw consent, forgotten right, and portability at any time. Foreign medical big data market is gradually maturing Data source crunchbase, cbinsight arterial network mapping By summarizing, it can be found that many foreign companies mainly provide PaaS services (platforms and services) for medical service providers. Provides decision support with artificial intelligence or machine learning after turning large amounts of data into usability data. In addition to startups, there are also many giants involved in the field. Including major companies in the field including GE Healthcare, Truven Health Analytics, UnitedHealth Group, Philips, Premier, Inc, SAP, SAS, Siemens Healthineer, Tableau Software, Inc, Xerox, Verisk Analytics, Allscripts, Sparx IT Solutions, Companies such as Oracle. As with the domestic market, the most accurate medical market in the cancer market is the fastest growing field. The reason for the recent rise of oncology clinics is that the treatment of tumors is very complicated. And the data can transform this process, making the medical staff's good treatment plan a replicable template rather than just the experience. Tumor big data is of great value. Tumor diseases are diverse, and every type of organ can occur. EMR, digital imaging systems, omics data, etc. can generate a large amount of data. Tumors have always been the top priority of pharmaceutical companies. In 2017, the global total of TOP25 oncology drugs achieved sales of 79 billion US dollars. The report from IQVIA predicts: "In 2022, the global cancer therapeutic drug market will reach 200 billion US dollars, with an average growth of 10-13% in the next five years, to the US market in 2022. It will reach $100 billion, an average increase of 12-15%." The expenditure on cancer drugs is mainly concentrated in a few treatments. The top 35 drugs account for 80% of the total expenditure, while more than half of the cancer drugs have annual sales of less than 90 million US dollars. In the past 10 years, the listing price of new anticancer drugs has steadily increased. The median cost of new anticancer drugs in 2017 exceeded $150,000, while the annual cost of new anticancer drugs in 2013 was $79,000. There are two main business models for foreign startups. One is to charge medical service providers and insurance providers. Because of the decision-making support provided by big data, it can bring better medical results and increase efficiency and cost. With the future value of medical insurance and the payment based on results, both medical service providers and medical service payers are facing increasing pressure on fees. The customer base of such companies will also grow larger. The second is to provide services like Google, such as Flatiron, Tempus. Make money for free or provide cheap services and then make money through the data collected in the background. The biggest paying party for these data is the pharmaceutical company. Data is of great value to pharmaceutical companies, but regardless of the business model, pharmaceutical companies are potentially huge payers. Tumor cancer care costs are rising year by year, global cancer drug spending continues to grow, and treatment and supportive care spending reached $133 billion in 2017, compared to $96 billion in 2013. Although a large amount of patient data also has the potential to pay for the sales of pharmaceutical companies, the data companies in foreign countries have not yet realized the value of data to the sales side. At present, their main problem is how to occupy customers, especially in the market, there are already many powerful giants, such as IBM Watson and GEhealth. The second is how to meet compliance requirements in a tightly regulated market. It takes a certain amount of time to turn real-world data into real-world evidence. There is no doubt that pharmaceutical companies must participate in it. From the above inventory, it can be seen that pharmaceutical giants such as Roche have already seen a number of big data companies through investment mergers and acquisitions. Transforming pharmaceutical processes with big data is also an FDA-driven policy trend abroad. FDA Director Dr. Scott Gottlieb said in an oral report; "Clinical trial reform is imperative, and efficient and modern clinical trial design can speed up the launch of new drugs, if you are doing modern, evidence-based, and rigorous things, It ensures great efficiency and great protection for the FDA gold standard.†Currently, in order to promote clinical trial reform, the FDA has issued several guidelines for guidance. Including the recommended EDC system and interoperability with the EHR system, most of the cancer clinical trials may not be a placebo control. Second, with the application of real world data. Pharmaceutical companies still face greater risks in accordance with traditional research and development traditions. Once oncologists and other stakeholders can accurately track the treatment of cancer patients. If a particular treatment does not actually work, it does not seem to work for a subset of patients (possibly molecularly defined). The efficacy or relative efficacy of the drug can be determined quickly and accurately. This may immediately ruin the story of pharmaceutical companies and blow their beliefs. The story of the panacea that pharmaceutical companies boast may be eclipsed in the face of real-world data. Is there any confidence in pharmaceutical companies? A report from Accenture pointed out that among the best-selling top10 drugs, those who took them only played a role in 4%-25% of patients taking the drug. Therefore, it is necessary for pharmaceutical companies to predict real-world efficacy before going public, because limiting pharmaceutical companies' commercial drugs may no longer be FDA-based clinical trial data review, but whether it can pass real-world validation by oncology clinical data companies. . The domestic market is committed to transforming data into value in the future Data source: Enterprise search for arterial network mapping Due to the late start of digital health in China, many domestic enterprises have solved the problem of data collection. Focus on structuring data through a unified data standard. In China, policies and capital markets are very promising for the development of medical big data. In 2016, the General Office of the State Council issued the “No. 47 Document†to promote and standardize the application of health care big data. Health big data is listed as an important basic strategic resource of the country, and it is a national control resource like oil and electricity. The Arterial Network has also reported that between 2013 and 2015, a total of 58 policies related to health care big data were issued by local authorities. In terms of data standardization, the state has successively issued guidance documents such as “E-medical Medical Record Basic Architecture and Data Standards†and “Electronic Medical Record Sharing Document Specificationâ€. In addition, many hospital information systems participate in the interoperability maturity assessment, laying the foundation for future data applications. It is worth noting that the policy also explicitly mentions research and formulation of government support policies, and provides necessary support for the development of health care big data applications from the aspects of finance, taxation, investment, and innovation. In terms of policy support, foreign FDA has made great efforts to promote data structuring and standardization. The federal government has spent more than $28 billion over the past decade to implement digital electronic health records. In China, there is no unified standard, and domestic policies cover a wider range. In addition to promoting data sharing and data standardization issues, it also includes many medical big data talent training, and encourages the guidance of state capital and social capital to participate in medical big data development. Wait. In the domestic market, medical big data companies started later than foreign companies, but the upstream and downstream layout has been formed in the entire industry chain. Some analysts pointed out that in the past six months, there have been many financing projects in the domestic medical big data field, but the current domestic application status is still far from the ability to build data analysis and analysis platforms. The platform analysis ability of data analysis is weak; more concentrated in a single direction, the integration of diversified data analysis intentions is less; the value presentation and value circulation do not form an ecological cycle. The domestic market is focused on data collection. According to the estimated industry development trend, data analysis is the value of big data. IQVIA's forecast report shows that analytics services dominate the market in different big data components and services, with revenues of $5.8 billion in 2017 and a compound annual growth rate of 22.3% for the 2017-2025 forecast. . After data is valued, computing tools will be used in the future to help intelligent decision making, enabling tools that track patient information and provide quick feedback. The main payers of medical big data are mainly divided into six: consumers, enterprises, insurance companies, governments, hospitals and pharmaceutical companies. In the short term, insurance companies and pharmaceutical companies have the strongest willingness to pay for hospitals. There are applications that represent companies that are trying to experiment with big data. Chinese medicine and biotech companies such as Hengrui Medicine, Hualing Medicine, and Tiantan Bio have already cooperated with Oracle on medical big data. Among the startup companies, for example, Sipai Network has cooperated with nine first-line foreign-funded pharmaceutical companies in market positioning, analysis of pharmacoeconomic evaluation, telemedicine , grading medical treatment, and smart medical care, and has formed scale income. We believe that the demand for medical big data, especially tumor big data, is obvious in hospitals, governments and enterprises, but it is still conservative at this stage. Domestic artificial intelligence, deep learning, natural language processing and other technologies are also continuing to develop. Domestic medical big data companies have begun to focus on different fields and gradually widened the gap. Under the policy push and the capital is optimistic, the market will also mature. Attachment: Foreign medical data company profile: Health Catalyst Health Catalyst is a US medical data management analytics service that helps different healthcare organizations analyze and manage clinical, financial, and operational data to increase productivity, reduce waste of health resources, and standardize medical processes. HealthCatalyst has also developed a series of new analytics applications that help different teams identify the best practice models, make the necessary interventions for clinical, financial and operational, screen out specific, individualized problem-solving solutions, and optimize clinical, financial and Operational results. In the past, the health catalyst was only responsible for collecting data and building its own large database, but later the health catalyst was transformed into a structured, standardized data that would satisfy the needs of different users. In July of this year, it acquired medcity and expanded its leadership in the field of medical big data. Health Catalyst has added more than 100 customer resource bases to include employers in 21 states and territories, health plans, 75 health systems, including more than 1,000 hospitals and more than 185,000 physician groups and extended medical care. The provider of the facility supports more than 75 million patients. The combined company will address many of the most pressing issues in large medical distribution networks as they seek to improve quality and reduce community patient care costs. Flatiron health Flatiron Health is a healthcare technology company that develops software that connects community oncologists, academics, hospitals, life science researchers, and regulators on a shared technology platform. Its main product, the Flatiron platform, a web-based business and clinical information data platform that integrates and structures patient population information systems to generate patient views, provides business intelligence analysis, resource utilization, marketing, treatment models, network management, and Research and clinical trials, and allow cancer care providers and life science companies to track indicators related to cancer treatment, managing cancer patient compliance in compliance situations. And ask custom questions about their data. Flatiron Health also offers OncoCloud, a suite of software and services, OncoEMR, which provides cancer care modelling tools for cancer care providers. That is to say, it is possible to intelligently generate a cancer care model recommended by the medical staff for the medical staff to select. OncoBilling, a practice management system that integrates OncoEMR integration with OncoAnalytics. Contains surface-operable dashboard design data insights. OncoTrials, a tool platform for managing cancer clinical trial projects for community cancer trials. In addition, the company provides a real-world evidence platform for life science oncology research, providing an electronic health record (EHR) data platform for academic medical centers and hospitals. Flatiron Health serves hospitals, doctors and patients in the United States. It has strategically partnered with the National Cancer Institute to explore how real-world evidence extracted from data from unidentified patients collected at care sites can be used for clinical trial design and prospective studies. Previously, it had received investments such as Googl and Roche. In February 2018, it was currently acquired by Roche for a total price of $2.1 billion. REDOX health field modern API interface Redox accelerates the development and release of medical software solutions through a full-service integrated platform to exchange data securely and efficiently. Establish an industry-standard platform for seamless interoperability between healthcare IT staff and healthcare systems. Often, sharing patient data across systems is a complex, manual, and time consuming process. Founded in 2014 by former Epic Systems game engineers, Redox integrates with all major EHR systems by connecting to existing health system infrastructure, significantly reducing implementation time by eliminating system considerations and configuration requirements. The API platform also allows applications to push patient data into and out of EHR to create an expanded, comprehensive patient health record. As a result, Redox customers experience less disruption, see the continuity of patient care benefits, and achieve faster return on investment. To date, more than 120 applications use Redox's integrated platform, including care coordination, telehealth, drug adherence, patient involvement, chronic care and disease management solutions. In the past year, the redox network has grown significantly, processing more than 600,000 clinical information per day. The company's CEO said that although there are some policies and specifications for clinical data standardization, I think that with our system, our customers can wait longer. Syapse Syapse works with health systems to implement precise medical programs in hospitals and care facilities that enable oncologists and nurses to provide personalized treatment to every patient who needs it. Syapse has developed an accurate medical software platform that enables academic and community healthcare providers to implement and extend precision medical programs. The Syapse Precision medical platform captures clinical data, genome and other molecular data, biomedical knowledge, and integrates relationships between these data, and integrates complex genomic and clinical data to provide decision support to clinicians for diagnosis, treatment, and Patient follow-up. The Syapse Oncology application, which provides oncologists with recommendations for treatment options based on molecular contour data of the patient's clinical history. Syapse PGx is an application that enables clinicians to incorporate pharmacogenomics into routine clinical workflows when ordering drugs through EMR. Syapse is the solution platform for precision medicine. In the past, cancer treatment patients needed various tests, wasted a lot of time, and delayed the timing of treatment. Syapse used biomarkers and genetics to customize solutions for patients. Syapse's team designed a collaborative ecosystem that allows healthcare professionals to make treatment decisions based on complete patient information. Syapse recently partnered with pharmaceutical company Roche to develop new software and analytics solutions for healthcare providers so they can implement precision medicine on a large scale. Oncology Oncology provides evidence-based, technology-driven use management methods for health programs. Focus on oncology. Used by doctors to support more than 2.5 million health program members in the United States and Puerto Rico, Oncology's e-Prior licensing platform is updated daily to accurately reflect more than 6000 anti-cancer treatments for cancer treatment programs in all cancer types and stages, including chemotherapy, radiation therapy, precision medicine, Targeted therapy and supportive care. The Cancer Analysis Company provides a cancer patient management solution. Its oncology management services include pre-authorized services; clinical decision support, physician education and expert peer review services; oncology network assessment and optimization consulting; and performance reporting and analysis services. The company offers MATIS, a clinical decision support software for medical programs and suppliers in the United States and Puerto Rico, and enhanced evidence-based oncology analysis methods for assessing cancer treatment and welfare management. GNS health GNS Health focuses on advancing and applying industry-scale data analytics to empower key healthcare stakeholders to address complex care, treatment and cost challenges. GNS Health's team consists of a multidisciplinary team of physicists, actuaries, geneticists, engineers, business people, and computer scientists who are passionate about extracting evidence of how the healthcare industry works and what it serves. GNS Health has developed machine learning and data analysis technologies designed to improve healthcare treatment and practice through computer modeling. The head of the company said: Pharmaceutical companies believe that GNS technology may help improve drug development efficiency. Because the analysis method from GNS health can analyze infinitely close patient-related data as well as their disease status, including gene sequencing information and health records, biological information and. The success and failure of existing therapies, as well as data on drug testing. GNS Health's machine learning and artificial intelligence-based analysis tools allow pharmaceutical companies to test multiple versions of a particular drug faster, more accurately, such as for multiple sclerosis versions, to find the best fit for a single patient. drug. The company's CEO said: "We can now simulate the effects of multiple sclerosis drugs No. 3 and No. 5 and No. 8 on the clinical outcome of this patient in a new patient, and even the impact on the total cost of treatment for this patient." Currently GNS health and Roche cooperate.
Rhodiola extract is the root extract of Rhodiola, which is sweet and bitter. The main ingredients are salidroside, tyrosol and losevin, which have the effects of enhancing immune function, protecting cardiovascular and cerebrovascular system, anticancer and antidepressant. Rhodiola extract is a natural extract extracted from Rhodiola crenulata, which is refined, concentrated and dried. It is very soluble in water, methanol, ethanol and ether. It is stable to heat. Rhodiola mainly contains phenylpropanoids and flavonoids. Its unique active chemical constituents are phenylpropyl esters, rosavin (the most active), rosin, rosarin, rhodiolin, salidroside and its aglycone [1], namely p-tyrosol. Only Rhodiola rosea contains rosavin, rosin and rosarin.
Product Name: Rhodiola Rosea Extract
Other name : Rhodiola crenulata
Used Part: Root
Specification: 5%
Appearance: Red brown powder
Active Ingredient: Rosavin, salidroside
Test Method: HPLC
Extraction type: Liquid-Solid Extraction
Shelf life: 2 years
Rhodiola rosea is a remarkable herb that has a wide and varied history of uses. It is thought to strengthen the nervous system, fight depression, enhance immunity, elevate the capacity for exercise, enhance memory, aid weight reduction, increase sexual function and improve energy levels. It has long been known as a potent adaptogen. Adaptogens are substances that increase the body's overall resistance and help to normalise bodily functions.
1. Rhodiola Rosea P.e can be Anti-aging, anti-anoxia, anti-fatigue and anti-stimulus effect.
1.Rhodiola Rosea P.e is Applied in food fields, it is widely used as functional food additive;
Why choose us?
Product Recommended
Wolfberry Extract,Ginkgo Biloba Extract,Rhodiola Rosea Extract,Ginseng Extract Xi'an Tian Guangyuan Biotech Co., Ltd. , https://www.tgybiotech.com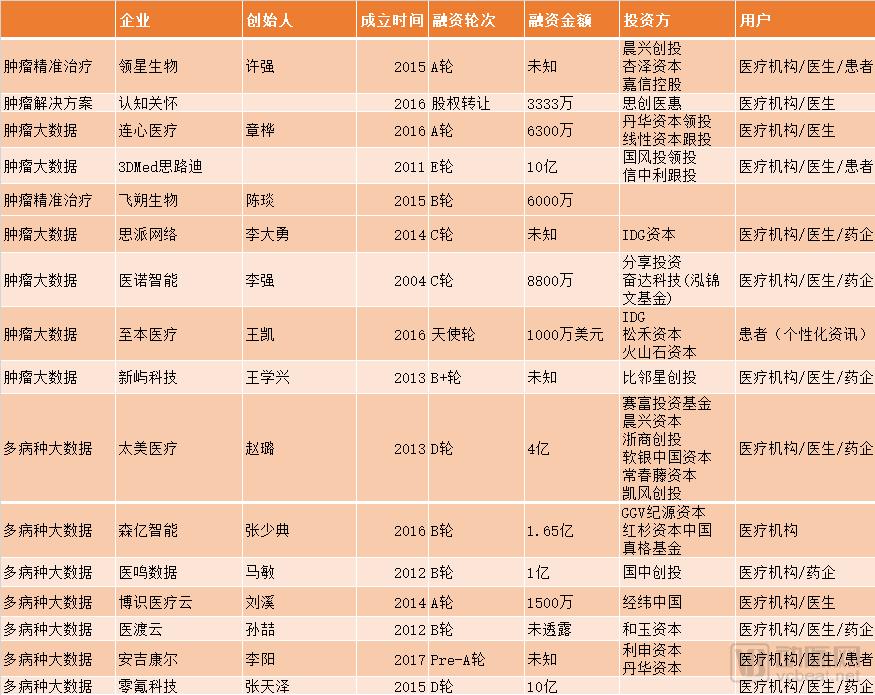


2. Rhodiola Rosea P.e is used as tonic and sexual elixir for both man and woman in china.
3. Rhodiola Rosea P.e Significantly allow for improvements in athletic performance.
4. Rhodiola Rosea P.e Effects from boosting immune function to enhancing memory to improving recovery from exercise.
5. Rhodiola Rosea P.e Reduce hyperglycemia visibly.
6. Rhodiola Rosea P.e can Be effective for improving mood and alleviating depression.

2.Rhodiola Rosea P.e is Applied in health product field, it can be used as raw material in health food, the purpose is to enhance immunity and resist melancholy;
3.Rhodiola Rosea P.e is Applied in cosmetics fields, it is widely used to delay aging and compact skin;
4.Rhodiola Rosea P.e is Applied in pharmaceutical field, it is widely used to treat cardio-cerebrovascular disease.
